Winter 2020
The Endocannabinoid System — A Primer
This relatively newly discovered system orchestrates a surprising number of biological functions.
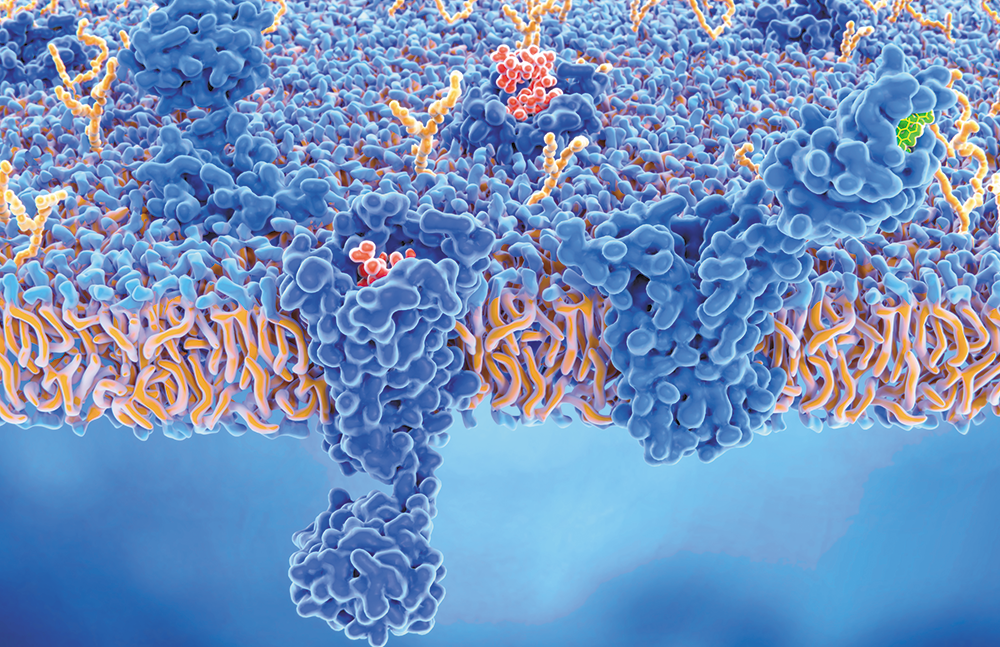
Health care professionals are well versed in most body systems, eg, the nervous, digestive, and immune. The goal of each of these systems is to maintain conditions within a narrow range—in other words, to keep the system in homeostasis. But what keeps all these systems working together in balance? The endocannabinoid system (ECS)—a relatively newly discovered and underrecognized collection of messengers, cannabinoid receptors, and related enzymes.
As the body of ECS science grows, so does the number of components identified. Found in all vertebrae species, the role of the ECS is to maintain homeostasis, which can be summarized as “relax, eat, sleep, forget, and protect.”1
Because the ECS has important implications for myriad functions and conditions, including memory, pain, stress, and reproduction, it’s considered the core of the mind-body connection. Interestingly, it was discovered by accident as scientists investigated how cannabis and its component cannabinoids (one type of the active constituents found in cannabis plants) influenced the nervous system; hence the name includes “cannabinoid.” The potential functions of the ECS are broad because of the complexity of the system and the potential influence over other systems and physiologic processes.
To understand the ECS, it’s important to have knowledge of the history of its discovery, its component parts, its potential functions and mechanisms of action, and how external factors can help or harm it.
History of the ECS
It’s difficult to pinpoint the origin of the term ECS. A review of historical literature first mentions the isolation of a phytochemical classified as a cannabinoid—cannabinol—in the late 19th century.1 During the late 1800s and early 1900s, cannabis was among the most commonly prescribed “patent medications” used to treat a wide array of conditions. Its popularity sparked research projects around the globe.2 In 1940, a second cannabinoid, CBD, was discovered in a lab at the University of Illinois, but the body system in which this messenger played a role had not yet been discovered.3 Research into cannabinoids then slowed because cannabis was removed from the United States Pharmacopoeia, an event that cast a shadow of doubt on the health benefits of cannabis.2,4 In 1964, Raphael Mechoulam, PhD, isolated yet another cannabinoid, THC. His work focused on investigating how cannabis functioned in the brain, including how it exerted its psychoactive effects and its role in memory, pain, depression, and appetite.5
In 1988, the first cannabinoid receptor, CB1, was identified in the brains of rats and then of humans, and it was also discovered that these receptors were more plentiful than any other receptor in the brain.6,7 In 1992, a global team of researchers discovered an endogenous chemical messenger, the first endocannabinoid—similar in structure to the phytocannabinoid THC—that interacted with the CB1 receptor. They named it anandamide, after the Sanskit term for “bliss,” and today, N-arachidonylethanolamide (AEA) is still commonly referred to as “the bliss molecule.”8 In the 1990s, researchers uncovered an additional receptor, CB2, and a second endocannabinoid, sn-2-arachidonoylglycerol (2-AG).9,10 However, it wasn’t until 2000 that the ECS was first mentioned in the title of a peer-reviewed article.11
In short, as scientists worked to uncover the metabolic pathways of phytocannabinoids and then of endocannabinoids, they came upon a previously unknown molecular signaling system within the body that they discovered was involved in regulating a broad range of biological functions—what’s now known as the ECS.
Components of the ECS
Following are the elements of the ECS that are most understood to date.
ECS Messengers
Endocannabinoids are molecules produced by the body that bind to and activate the cannabinoid receptors. They’re produced in response to changes sensed by the body in order to “talk” to the inside of cells and tell them what to do to maintain homeostasis.
There are two major endocannabinoids (also called ECS ligands): AEA and 2-AG. AEA and 2-AG are released from phospholipids embedded in cell membranes when they are needed. Because they are lipid based, they’re produced on demand and not packaged and stored for later use like many other biological messengers.12
ECS Receptors
Cannabinoid receptors sit on the surface of cells and “listen” to conditions outside the cell. They transmit information about changing conditions to the inside of the cell, kickstarting the appropriate cellular response. Cannabinoids, both endo- and phytocannabinoids, interact with these receptors, causing a cascade of reactions.
CB1 is the most abundant G protein–coupled receptor (GPCR) expressed in the brain. GPCRs are considered the “inbox” for messages sent between cells. CB1 receptors are also found in the peripheral nervous system, adipocytes, hepatocytes, and, to a lesser extent, in musculoskeletal tissues.13
CB2 is also a GPCR primarily located on immune cells and structures such as the spleen, tonsils, and thymus glands and localized on monocytes, macrophages, B-cells, and T-cells; CB2 receptors are also found in the peripheral nervous system, gastrointestinal system, and central nervous system.14,15
Metabolic Enzymes
Because endocannabinoids function in homeostatic fashion, they are broken down as soon as they deliver their messages to the receptors. The enzymes that catabolize the two main cannabinoids are embedded in the cell membranes. AEA is broken down by fatty acid amide hydrolase (FAAH), and 2-AG is broken down by monoacylglycerol lipase (MAGL).16,17
Supplementary Components of the ECS
Research into the ECS is still in its infancy, but knowledge is growing at a rapid pace. Understanding of the “classic” ECS described above has been expanded to include secondary receptors, ligands, and metabolic enzymes. While not the focus of this discussion, these secondary compounds may produce synergistic effects by inhibiting the breakdown of ligands; this prolongs their action.
How the ECS Functions
The three pillars of the ECS—endocannabinoids, cannabinoid receptors, and metabolic enzymes—are found in almost every major system of the body. When an imbalance is detected within the body, cells synthesize endocannabinoids that interact with cannabinoid receptors. This stimulates a chemical response that works to bring the body back to homeostasis. Then the endocannabinoids are hydrolyzed to prevent an overcorrection of the system. Because the list of potential ECS functions is beyond the scope of one article, following is a discussion of two examples of the major functions.
Endocannabinoid Regulation of Nociceptive Pain
Imagine a physical injury, such as a stubbed toe. There’s a cascade of physiological responses that start the healing process but that also may cause pain. There’s an increase in blood flow to the area and immunomodulatory messages are sent there. In the brain, electrochemical signals are sent between neurons. As the neurons become overloaded, the brain senses pain. In order to bring the body back into balance, cells in the brain release AEA. When AEA binds to CB1 receptors, the release of inhibitory neurotransmitter is stimulated, resulting in reduced pain signals and a return to homeostasis.18 Significantly, this is a retrograde signal specific to endocannabinoids that allows the “receiving” neurons to regulate how much information they accept from overactive “sender” neurons.19
Endocannabinoid Regulation of Inflammation
2-AG is the most prevalent endocannabinoid in the human body. Due to its high expression in peripheral immune cells, it plays a large role in anti-inflammation through immune suppression. While inflammation is a natural protective reaction the body has to infection or physical damage, persistent inflammation can be damaging. When the inflammatory response lasts too long, the ECS kicks in, limiting the immune system’s inflammatory signals.
Consider an immune response triggered by infection. First, the immune cells detect the presence of a foreign material, and proinflammatory molecules, which recruit other immune cells to fight the infection, are released. At the same time, 2-AG is released, binds to CB2 receptors, and starts a cascade of reactions to balance the immune response, which prevents excessive inflammation. An emerging body of research suggests that if the ECS is not functioning optimally, it may lead to or exacerbate autoimmune conditions.20
The Role of Cannabis in Regulating the ECS
As with other physiologic systems, there are exogenous factors that can influence the ECS. Of significant interest is cannabis and the derivative phytocannabinoids. Plant cannabinoids such as THC have clinical and psychoactive effects because of their interactions with the cannabinoid receptors. For instance, THC causes a euphoria or “high” because it activates CB1 receptors within the brain, but not in the same way as does AEA.21 First, THC attaches to CB1 receptors more tightly than does AEA; second, the internal regulatory mechanisms such as FAAH that control the amount of AEA in the system don’t work on THC. Therefore, THC exerts a stronger effect and stays in the system longer to produce more significant psychoactive effects.22 Moreover, other cannabinoids such as CBD can interact with more than one receptor type or even have different metabolic effects. For instance, CBD’s best-known action is actually as an inhibitor of FAAH, which means CBD slows the degradation of natural AEA without interacting with any cannabinoid receptors.23
Other Factors That May Influence the ECS
As research into the ECS expands, the role of suboptimal system function is at the center of potential therapeutic interventions. In fact, some scientists and clinicians believe that decreased ECS activity or “tone” is at the center of migraine, fibromyalgia, irritable bowel syndrome, and autoimmune conditions; this decreased ECS tone is a condition now referred to as clinical endocannabinoid deficiency syndrome (CEDS).20
The following are three proposed mechanisms by which to correct CEDS (these also may be important for patients who do not have a diagnosis related to CEDS but who suffer from decreased ECS tone)24:
• augmenting ECS messenger biosynthesis (increase AEA and 2-AG);
• decreasing ECS metabolic enzyme activity (influencing the action of FAAH and MAGL); and
• increasing or decreasing cannabinoid receptor density or function.
Cannabis and phytocannabinoids offer ways to improve ECS tone via some of these mechanisms and may seem like the lone solution; yet there are studies, to be discussed, suggesting other interventions that may upregulate a dysfunctional ECS.
Pharmaceutical Drugs
Ibuprofen and other over-the-counter nonsteroidal anti-inflammatory drugs appear to block FAAH, stopping the breakdown of AEA, which may influence pain perception.25
Acetaminophen may also block FAAH to stop the breakdown of AEA and increase the activity in neuronal synapses to decrease pain.26
Glucocorticoids have acute and chronic effects on the ECS. Glucocorticoid receptors overlap with CB1 receptors throughout the body. When administered acutely, glucocorticoids may increase ECS messenger synthesis in parts of the brain. Chronic administration may downregulate the expression of CB1 receptors in the brain, resulting in decreased ECS tone.24
Dietary Supplements
Some studies show that dietary supplementation with linoleic acid (precursor to arachidonic acid, a polyunsaturated fatty acid), increased blood levels of AEA and 2-AG. However, excessive arachidonic acid may throw the ECS out of balance.27
Probiotics and prebiotics (eg, Lactobacillus acidophilus, oligofructoses) may increase CB1 and CB2 receptor expression and have been shown to reduce pain behavior in mice.28
Herbal Remedies
Other plants may have what are considered “cannabimimetic” effects—they act like cannabis in the body. For instance, flavonoids from red clover, soybeans, and some teas may inhibit FAAH even when consumed in small amounts. Curcumin may elevate ECS tone.24 The principal terpenoid in black pepper, beta-caryophyllene, binds to CB2 receptors and exerts anti-inflammatory effects.29
Mind-Body Connection
Chronic stress results in chronic elevation of endogenous corticosteroids, reducing AEA levels throughout the ECS, and other stress factors influence levels of 2-AG. Clinical anecdotes suggest that stress-reduction techniques such as meditation, yoga, and deep breathing may improve ECS tone by exerting a cannabimimetic effect that reduces the overabundance of stress messengers.30
Lifestyle Modification
Increased food intake and adiposity may elevate AEA and 2-AG expression. Weight loss from caloric restriction may reduce CB1 receptor expression and bring the ECS back into balance.24
Rat studies show that exercise may affect ECS tone; however, there’s a difference between voluntary exercise and forced exercise. While both increase levels of AEA and 2-AG in the short term, only forced exercise—defined as exercising harder than a “usual” rate—led to predictable increases in endocannabinoids.24
Like exercise, alcohol intake affects the ECS based on acute or chronic intake. When taken only occasionally in small amounts, ethanol intake may enhance ECS tone. However, chronic ethanol consumption and binge drinking likely decreases the appearance of CB1 receptors, thus contributing to ECS dysfunction.31
Research doesn’t provide a clear understanding of how nicotine affects the ECS. While one study shows that it increases AEA and/or 2-AG in one area of the brain, another study shows that it decreases the endocannabinoid expression in another area of the brain.24
Factors such as caffeine, massage therapy, aromatherapy, and other manipulation and/or lifestyle interventions may affect the ECS and should be considered as part of a plan to increase ECS tone if not otherwise contraindicated. The final word on improving or optimizing ECS function has not been written, and it’s important to experiment with any nonhazardous approaches clients are willing to attempt.
Cannabis
How cannabis and its elements affect the ECS is as complex as the system and the plant combined. Research has yet to define the difference between acute and chronic dosages, making it difficult to offer recommendations about improving ECS tone. Research shows that acute administration of THC to rodents decreases the sensation of pain, but long-term administration of THC results in desensitization to the pain relief.32 Because cannabis is more than THC, and much of the research has been done with synthetic cannabinoids or single-molecule isolates, the true impact of cannabis on the ECS is yet to be determined.
Conclusion
The ECS is an emerging, complex physiological system that involves multiple body processes. The endogenous messengers, receptors, and metabolic enzymes that have been identified have provided the basis for understanding how the ECS is involved in overall homeostasis. However, more research—preclinical and clinical—needs to be done. That said, the mantra of clinical cannabis use remains true: Try it, keep detailed records of the effects, adjust accordingly, and report the findings.
Moreover, a comprehensive lifestyle modification plan may be the key to improving ECS tone. By reviewing the various factors mentioned earlier—supplement use, stress level, diet, exercise, and alcohol and nicotine use—it may be possible to develop a series of steps to improve ECS tone with or without cannabis.
Bonnie Johnson, MS, RDN, is a certified food industry consultant, speaker, and certified cannabis consultant. As a consultant, she works with the food and cannabis industries to bring science-based education to health care professionals and category-changing products to market.
TIMELINE OF DISCOVERY
End of 19th century: The first plant cannabinoid (phytocannabinoid) is isolated: cannabinol.1
1940: Roger Adams, PhD, of the University of Illinois, discovers a second cannabinoid: CBD.3
1964: Raphael Mechoulam, PhD, at Hebrew University of Jerusalem, isolates another cannabinoid: THC.5
1988: The first cannabinoid receptor, CB1, is discovered in the brain of rats and confirmed in humans. Researchers show that CB1 receptors are the most prevalent receptors in the brain.6,7
1992: Mechoulam and a global team of researchers discover an endogenous cannabinoid, anandamide, which interacts with CB1 receptors in a manner similar to THC.8
1993: A second cannabinoid receptor, CB2, is found in the heart, blood vessels, kidneys, bones, intestines, spleen, reproductive organs, and lymph system.9
1997: A second endocannabinoid, 2-AG, is discovered.10
2000: The first time the term “endocannabinoid system” appears in peer-reviewed literature.11
References
1. Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147(Suppl 1):S163-S171.
2. Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T. 2017;42(3):180-188.
3. Adams R, Hunt M, Clark JH. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. J Am Chem Soc. 1940;62(1):196-200.
4. Galliher JF, Walker A. The puzzle of the social origins of the Marihuana Tax Act of 1937. Soc Probl. 1977;24(3):367-376.
5. Mechoulam R. Marijuana: Chemistry, Pharmacology, Metabolism and Clinical Effects. New York, NY: Academic Press; 1973.
6. Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605-613.
7. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561-564.
8. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946-1949.
9. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61-65.
10. Stella N, Schweitzer, P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773-778.
11. Piomelli DA, Giuffrida A, Calignano A, Rodríguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000;21(6):218-224.
12. Di Marzo V. ‘Endocannabinoids’ and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim Biophys Acta. 1998;1392(2-3):153-175.
13. Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299-318.
14. Onaivi ES. Commentary: functional neuronal CB2 cannabinoid receptors in the CNS. Curr Neuropharmacol. 2011;9(1):205-208.
15. Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160(3):467-479.
16. Bisongo T, De Petrocellis L, Di Marzo V. Fatty acid amide hydrolase, an enzyme with many bioactive substrates. Possible therapeutic implications. Curr Pharm Des. 2002;8(7):533-547.
17. Dinh TP, Carpenter D, Leslie FM, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99(16):10819-10824.
18. Sallaberry CA, Astern L. The endocannabinoid system, our universal regulator. J Young Investig. 2018;34(6):48-55.
19. Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 2012;18(2):119-132.
20. Russo EB. Clinical endocannabinoid deficiency reconsidered: current research supports the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant syndromes. Cannabis Cannabinoid Res. 2016;1(1):154-165.
21. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):E833.
22. Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 2016;96(4):1593-1659.
23. Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515-527.
24. McPartland JM, Guy GW, Di Marzo V. Care and feeding of the endocannabinoid system: systematic review of potential clinical interventions that upregulate the endocannabinoid system. PLoS One. 2014;9(3):e89566.
25. Fowler CJ, Janson U, Johnson RM, et al. Inhibition of anandamide hydrolysis by the enantiomers of ibuprofen, ketorolac, and flurbiprofen. Arch Biochem Biophys. 1999;362(2):191-196.
26. Högestätt ED, Jönsson BA, Ermund A, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280(36):31405-31412.
27. Alvheim AR, Malde MK, Osei-Hyiaman D, et al. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring). 2012;20(10):1984-1994.
28. Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35-37.
29. Gertsch, J, Leonti M, Raduner S, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105(26):9099-9104.
30. McPartland JM, Giuffrida A, King J, Skinner E, Scotter J, Musty RE. Cannabimimetic effects of osteopathic manipulative treatment. J Am Osteopath Assoc. 2005;105(6):283-291.
31. González S, Cascio MG, Fernández-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954(1):73-81.
32. Suplita RL 2nd, Eisenstein SA, Neely MH, Moise AM, Hohmann AG. Cross-sensitization and cross-tolerance between exogenous cannabinoid antinociception and endocannabinoid-mediated stress-induced analgesia. Neuropharmacology. 2008;54(1):161-171.
