Fall 2020
Ask the Expert: How Does Medical Cannabis Help Parkinson’s Disease Patients?
An individualized approach focuses on improving quality of life.
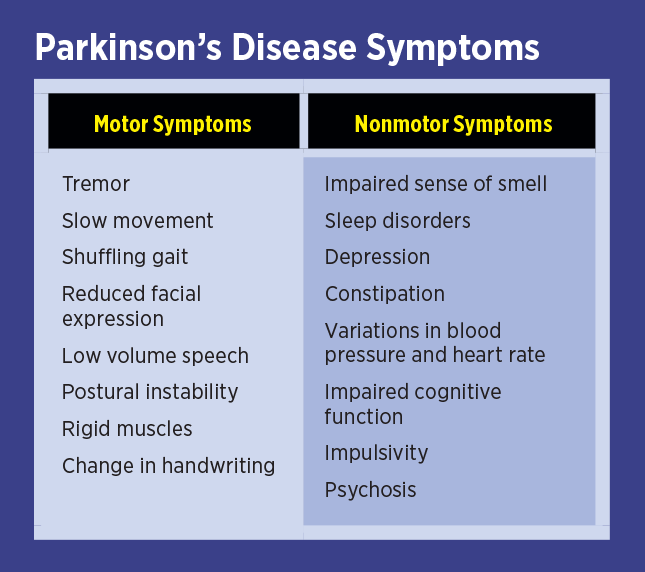
Parkinson’s disease (PD) is a neurodegenerative condition caused by a progressive loss of dopamine-containing nerve cells in a part of the brain called the substantia nigra. Patients with PD suffer from a variety of motor and nonmotor symptoms (see table below), many of which respond to conventional and alternative therapies. PD is diagnosed by taking a history, performing a physical examination, and ruling out Parkinsonism. Parkinsonism may look like PD, but its causes include toxins, hydrocephalus, stroke, or other forms of brain degeneration. There’s no single test for diagnosing PD; it’s a clinical diagnosis, one made by the astute practitioner based on information provided by the patient.
Not all PD patients present in the same way. For example, some may have prominent tremors, whereas others have no tremor at all. Early in the disease, some will present only with reduced sense of smell and sleep disturbance. Diagnosing PD early and determining which symptoms require which type of therapy represents the art and science of medicine. Naturally, preventing the development of PD or other degenerative diseases is preferable to simply treating the symptoms of the disease once they occur. With PD, the symptoms may only become evident after more than 50% of neurons in the substantia nigra have already died off.1
To see how cannabis may help a patient with PD, consider the recommendations described below.
Case History
A 78-year-old gentleman reported generalized fatigue and weakness for the past year. He complained of difficulty motivating himself to do much of anything. He’d always been quite energetic, working as an attorney in Albany, New York. He stayed active by golfing, visiting with friends, and traveling out of state. More recently, however, he and his family noted that he was slowing down and was unable to walk more than 50 yards without rest. He no longer enjoyed socializing and in general appeared less animated. Most people thought he either was experiencing side effects from his cardiac medications or was suffering from depression. Indeed, he had a history of coronary artery disease and had been taking prescription medications. However, his cardiac condition had been stable for years and no new medications had been introduced. He denied any pain in the back or legs, and a recent physical examination revealed stable cardiac function and no problem with circulation. He denied any shortness of breath or chest pain, but did notice reduced appetite and trouble sleeping. Laboratory testing revealed no abnormalities; there was no evidence of anemia or thyroid dysfunction and no vitamin deficiency or electrolyte imbalance.
Examination
I spent some time with the patient at his home. He described how he no longer enjoyed food and how during the past year his sleep was interrupted by restless movements. His mental status was clear, and he remembered recent and remote events. Although the content of speech was normal, his speech was low volume and monotone. He also seemed to be staring—there was a reduction in normal eyeblink frequency. His facial expressions and speech lacked emotion. We walked out to the back patio and I noted his movements were slow; his arm swing was reduced and his posture was flexed with his head pointing down. I also noticed that while his hand was resting at his side, he had a pill-rolling tremor—his left thumb and forefinger were moving back and forth opposite each other very slightly.
Diagnostic Impression
The pill-rolling rest tremor along with my other observations convinced me that the patient had PD. The other findings he and his family reported and those observed also could be explained by a diagnosis of PD.
The report of weakness would certainly go along with abnormalities in motor function, seen in patients with PD. Sleep disruption, which, in PD, affects the rapid eye movement (REM) phase, and loss of smell are both early features of the condition in many patients. These issues would certainly contribute to daytime fatigue and loss of appetite. The staring appearance and low volume speech also indicate the condition.
Treatment Considerations
Degenerative brain diseases such as PD and Alzheimer’s dementia that get worse over time progress more slowly by reducing oxidative and excitotoxic damage to neurons. And although there are some ways to decrease these damages, we need to do better. Cannabis is both a potent antioxidant and effective way to reduce excitotoxicity, which occurs when too much of the neurotransmitter glutamate overactivates neurons. Cannabis modulates neurotransmitter release and restores balance, or homeostasis, to this signaling process.
Leading up to 2003, a large body of preclinical evidence supporting cannabis use to ameliorate neurodegenerative diseases was the basis for US patent 6630507, which granted the US government the right to profit from cannabis as a medication. More recently, work by Sanchez-Ramos and colleagues has demonstrated how cannabinoid medicine helps patients with PD and other movement disorders.2
One of the obvious and debilitating features of PD is bradykinesia, or hypokinetic movement. This is the slowing of movement that can be seen in the arms, legs, and facial muscles. Patients want to move and have the strength to move but cannot get the muscles to act in a fluid, normal tempo. Sinemet (L-dopa-carbidopa) or similar dopamine replacement therapy may be very helpful for bradykinesia in the limbs and may restore animation to speech and facial expression. The evidence for cannabis use to improve bradykinesia in humans remains limited, although laboratory evidence abounds.3 In fact, cannabis may further slow movement, so caution is needed. Anyone who has experienced sudden inability to move after taking a dab or has experienced the less intense but still obvious effect called “couch-lock” can attest to the reduction in mobility associated with THC use. Interestingly, PD patients may also develop motor dysfunction in the form of overactive motor function. These hyperkinetic symptoms include tremors and dyskinesia (abnormal writhing movement). For hyperkinetic dysfunction, cannabis may be very helpful.
This case highlights the need for an integrative approach, one that incorporates elements of preventive medicine, conventional pharmaceutical therapy, and alternative treatment with cannabis.
Dosing and Discussion
In an older, cannabis-naïve patient, practitioners must proceed with cannabinoid therapy cautiously. Too much THC could exacerbate his already slow movements and create an unpleasant psychoactive experience. Additionally, when starting a long-term treatment protocol, it’s important to encourage a feeling of confidence and then demonstrate, through careful use, that the therapy will do no harm. The protocol—including likely target dose range, symptom targets, and time to achieve those goals—must be discussed up front. In this way, expectations are clear and compliance with treatment is encouraged.
For my patient, I would suggest beginning with a CBD program at bedtime to treat his sleep disorder. In one study of PD patients with REM sleep behavioral disorder, 75 mg of CBD eliminated symptoms in three patients, while another patient required 300 mg CBD at bedtime to reduce sleep disruption by one-half.4
Beginning CBD at bedtime can be challenging because low doses may have an alerting effect and cause trouble with sleep initiation. Therefore, I would start with 10 mg edible CBD when the patient gets into bed; this way, it should take effect after he’s asleep and prior to the first round of REM sleep, which usually begins about 90 minutes after sleep onset. Over two to three weeks, he can increase the dose to 75 mg or more, depending on results. With improvement in sleep, his daytime function may also improve.
The next phase of therapy might include addition of tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA) as a morning decoction or tea, made from flower with a 1:1 ratio. This would be a microdose made with a small amount of bud and boiling water. By pouring the water over the bud and steeping for only one to two minutes, there won’t be any significant decarboxylation or risk of intoxication. There’s anecdotal evidence that raw cannabis, with cannabinoids in the acidic form, may improve motor symptoms in PD.5 Furthermore, small amounts of acidic cannabinoids taken daily may have other tonifying benefits on overall health.
Finally, addition of THC may be advised. In this case, I would recommend a one-month slow titration starting with a few drops of a 19:1 (CBD:THC) tincture at bedtime (less than 3 mg total cannabinoids), increasing to one-quarter of a dropper over two weeks. Then add a late morning and afternoon dose of the same amount. This low-dose THC would enable the patient to acclimate to the THC and possibly enjoy increased appetite and improvement in well-being. Chances are he will need a bit more THC to experience real improvement in mood and appetite, and, therefore, I would switch him to a 1:1 tincture, again starting with just a few drops in the same manner as I did for the 19:1 preparation.
Taken together, the entourage would include CBDA, THCA, CBD, and THC. These elements would provide a reasonable chance of improving his current symptoms and possibly reducing his risk of disease progression. A sprinkling of terpenes and other cannabinoids would be additive—limonene to lift his spirits and energy level during the day and tetrahydrocannibavarin to provide another way to improve motor function and stave off metabolic syndrome. And, of course, exercise, proper nutrition, and spiritual support would round out the integrative to-do list.
I find that in caring for such patients, the most good can be achieved by first establishing their trust in the practitioner and treatment process. It may take time to see results, and you don’t want to give up prematurely. It is a wonderful thing to open the door to improved well-being and see the patient walk through, head high, with arms in full swing.
— Daniel P. Stein, MD, is a board-certified neurologist and principal physician at the Neurology of Cannabis Clinic in Sarasota, Florida. His published works include articles in the New England Journal of Medicine and the American Journal of Endocannabinoid Medicine. He’s a member of the American Academy of Neurology and the International Cannabinoid Research Society.
References
1. Burke R, O’Malley K. Axon degeneration in Parkinson’s disease. Exp Neurol. 2013;(246):72-83.
2. Catlow B, Sanchez-Ramos J. Cannabinoids for the treatment of movement disorders. Curr Treat Options Neurol. 2015;17(9):39.
3. Buhmann C, Mainka T, Ebersbach G, Gandor F. Evidence for the use of cannabinoids in Parkinson’s disease. J Neural Transm (Vienna). 2019;126(7):913-924.
4. Chagas MHN, Eckeli AL, Zuardi AW, et al. Cannabidiol can improve complex sleep-related behaviours with rapid eye movement sleep behavior disorder in Parkinson’s disease patients: a case series. J Clin Pharm Ther. 2014;39(5):564-566.
5. Whiteley N. CBD & Parkinson’s disease. Project CBD website. https://www.projectcbd.org/medicine/cbd-and-parkinsons-disease. Published July 12, 2017.